OfferGet 10% off on Dissertation, Assignments, Essays, Thesis
Metastatic colon cancer refers to the spread of cancerous cells from the colon to other organs in the body. The process by which these cells get dispersed to distant organs is called metastasis. According to the Memorial Sloan Kettering Cancer Centre (2016), up to 70% of people who suffer from colon cancer also develop a cancerous tumour in the liver. This paper explores the molecular mechanisms underlying the metastasis of colon cancer to the liver. Fidler (2005) and Valastyan & Weinberg (2011) based on their understanding stated that there are six sequential steps through which cancerous cells from a tumour located in a primary site (such as the colon) migrate to a distant site (such as the liver). These 6 steps are indicated in figure 1 and because they occur sequentially they are referred to as the ‘Invasion Metastatic Cascade’.
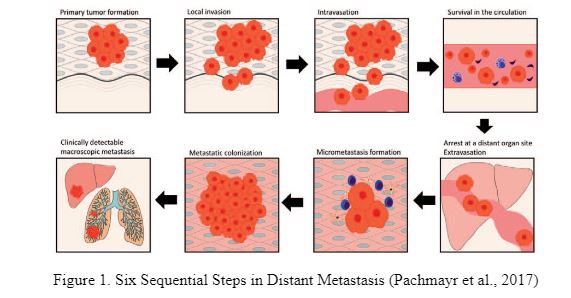
From figure 1, it is observed that the 6 steps include (i) local infiltration of the tumour cells into surrounding tissue (Invasion), (ii) trans-endothelial movement of the cancer cells into vessels (intravasation) (iii) survival in the circulatory system and transport through the blood, (iv) arrest/extravasation at the distant site, (v) micro metastasis and (vi) colonization of liver. Each of these steps is discussed below.
Invasion refers to the process by which the colon cancer cells move from the parent tumour into the surrounding tissue. A number of scholars have pointed that, the body tissues are composed of epithelial and mesenchymal cells. It needs to be mentioned that the epithelial cells attach to one another quite fast forming layers of cells that protect the body from the environment, mesenchymal cells occur single and can migrate (Samarsinghe, 2013) and Thiery et al (2009). In addition to these cells, tissues are composed of the extracellular matrix or ECM. Cells embedded in it forms the ECM molecules.
The cells attach to the ECM and to each other forming the tissues. The metastasis invasion process begins by the cancer cells untethering the bonds between Extracellular Matrix (ECM) cells that then enables them to migrate freely.
At the molecular level, there are several proteins that tether the cells to one another and to the surrounding tissues. These include immunoglobulins and cadherins which mediate intracellular adhesions and link the cells to the ECM as well. The most important protein is E-cadherin. According to Van Zijl et al., (2011) E-cadherin not only facilitates intracellular adherence it also outputs antigrowth signals that prevent cells that touch each other from growing further.
The cancer cells detach from the tumour in the colon and become spindly shaped. They stop expressing E-cadherin and hence are able to detach from other cells and from the ECM. They start expressing N-cadherin which is a protein that helps them to move in between blood vessels more efficiently whilst migrating (Samarasinghe, 2013). The changes that facilitate the tumour calls to migrate and thus invade distant organs is called Epithelial Mesenchymal Transition (EMT) (Thiery et al., 2009).
The development of signaling pathway models can be assumed as an important key molecular mechanism in EMT. WNT signaling leads to the determination of cells that also serves as an output in terms of establishing the relationship between the participating genes. WNT signaling has been used in a variety of cancers and loss of this signal or pathway is often assumed as the hallmark of human colorectal cancer. At the same time, aberrant WNT signals are closely associated with the colon cancer. Further findings revealed that the TGF-β signaling pathways often play an important yet paradoxical role in the progression of colon cancer. It is believed as a power inhibitor of colonic epithelial cells that also acts as a tumor suppressor. It is also assumed to be promoting the invasion, survival, and metastasis of the colon cancerous cells by acting as oncogene.
Xu and Pasche (2007) found that a common variant of the TGF-β is responsible for 3% of the colon cancer cases in the US. Novellasdemunt et al (2015) also found that WNT signaling play an important role in tissue homeostasis and the same has been implicated in a number of cancers.
According to Takanori et al., (2009) intravasation can be defined as the movement of cancerous cells through basal membranes and into the blood vessel. It is the next step in the series of carcinogenic events that leads cancer to shift from the primary position to a distant one. One of the genes that facilitates intravasation is urokinase (uPA) which is a protease. This gene also facilitates the emergence of different growth factors and the matrix metalloproteinases (MMPs) that exacerbates degradation of the ECM tissue and facilitating the invasion of tumour cells and intravasation (Berlth et al., 2014). Tremblay et al (2006) in regards to colon cancer found that the activation of E-selectin is an important procedure that regulates the extravasation of colon cancer. Mainly initiating ERK dependent mechanisms that contribute in terms of regulating the endothelial layer in a significant manner does this.
the transfer of tumour cells to the liver through the hepatic often includes the superior as well as inferior mesenteric veins and the portal vein. Fidler (1970 ) indicated that a key factor aiding metastasis through the circulatory system is the release of chemokines that are specific to liver and which attract the relevant chemokine receptors on the surface of the tumour cells. It needs to be mentioned that liver cells secrete cytokines that is also responsble for regulating the biochemical process and are recognised by these receptors on tumor cells. Such detection occurs through the blood stream. According to Pantel and Speicher (2016), blood actually provides a hostile environment for the circulation of tumour cells. Only 0.01 % of tumour cells passing through blood survive and result in distant metastasis. One of the killer factors is the high mechanical forces exerted on the tumour cells that is exerted by blood molecules (Joyce and Pollard, 2009).
This is particularly so when the tumour cells circulates within a narrow network of capillaries / intravascular structures of contractile tissues such as those of the heart where blood pressure forces them to change their spherical shape into cylindrical. This transformation of shape is lethal to tumour cells (Joyce and Pollard, 2009). However, metastatic tumour cells have developed capacity to survive these mechanical forces. They interact with neighbouring blood platelets and / or amongst themselves to form big emboli shaped cells that protect them from shear and stress forces (Yan and Juarsz, 2016). As per the Cancer Research UK (2017), it was found that colon cancer travels to the liver mainly because of the fact that blood circulates from the colon through the liver on its way back to heart. So, the colon cancerous cells escape into the circulation along with sticking in the liver as the blood passes through. This also leads to the formation of the secondary cancer as the blood passes to other parts of the body having cancerous cells. The second threat to the tumour cells as they move through the blood vascular system is immune surveillance exerted by the immune in the bloodstream. Tumour cells release cytokines, IL-1, 12 & 18 cells that in turn activate immune cells such as T-lymphocytes and natural killer or NK cells (Mehlen et al., 2006). The latter cells then ‘kill’ the tumour cells through Trail and / Natural Killer Group-2 (NKG2 processes) that is an immune receptor. Even here, tumour cells have developed the capability to elude immune surveillance by generating glycoproteins that protect them against attack from the immune / killer cells (Karkkainen et al., 2002). In this way, tumour cells have developed the capacity to survive in the hostile blood environment, facilitating extravasation to distant organs.
Extravasation is the process by which the tumour cells leave the blood circulation and then bind to the endothelium of the cells in the target organ (Sarvaiya et al., 2013). The vascular endothelium of the liver is fenestrated and does not have a strong basement membrane (Konstantinos, 2014). This facilitates the extravasation process. According to Kaplan et al., (2005) the tumour cells penetrate the porous vascular endothelium and interact with the hepatitis sinusoidal cells including the sinusoidal endothelial (SEC), Kupffer (KC), Hepatic stellate (HSC) and pit cells. Each of these cells play a crucial role in the progression of metastasis as they either facilitate the further invasion of the tumour cells into the liver or eliminate them. SEC’s are scavenger cells and perform a tumoricidal function (Lukanidin and Sleeman, 2012). However, under the influence of cytokines secreted by the invading tumour cells, SEC’s may in turn generate adhesion cells like E-selectin which facilitates the adhesion of the tumour cells to the endothelium and their further extravasation into the liver parenchyma. Shin et al., (2011) state that KC’s are also scavenger cells and plan an important role in arresting progress of tumour cells during the initial stages of extravasation.
However, KC’s may not succeed in killing the tumour cells completely. Moreover, KC’s secrete growth factors such as HGF, MMP-9 and MMP-14 which facilitate the proliferation of tumour cells (Shin et al., 2011). Cancer cells further secrete MIF that contain exosomes like TGF-b that activate the HSC’s (Bayon et al., 1996). This in turn recruits bone marrow derive cells (BMDs) to adhere to the tumour cells, further facilitating colonization. The pit cells also perform a tumoricidal action, contribute largely to hepatitis immunity and perform a strong antitumor action.
However, the tumoricidal action of the PC’s can be undone by the secretion of Angptl4 and MMP-1-2 proteins that contribute to vascular permeability (Nathan, 1986). In addition, tumour cells generate survival signals through secretions like galectin-3 which protects the cells from TRAIL – assisted apoptosis. They can secrete periostin which is another survival signal element and interact with hepatocytes to facilitate overt metastasis (Decker, 1989). Through these processes of extravasation occurring at the cellular level, tumour cells penetrate the liver and get ‘arrested’, adhering to the hepatitic endothelium leading to the next stage of metastasis.
According to Chin and Wang (2016), the arrival of the tumour cells at the target organ and the penetration of its tissue through extravasation is facilitated by the apriori creation of specific conditions. This is done by the primary tumour releasing growth factors such as P1GF, β (TGFβ), and proteins such as S100A8/-9 into the vascular system (Pasila and Lyden, 2009). These factors also recruit positive bone marrow progenitor cells to modulate the extra-cellular matrix at the liver. The liver tissue thus gets ‘primed’ even before the invasion of tumour cells, making it more susceptible for metastasis to occur. This process is called pre/micro metastasis.
Nguyen et al (2016) found that colon cancer metastasis to the liver is one of the major causes of cancer-associated deaths and genes and molecules governing this colonization are not characterised in a scientific manner. It was further found that the liver and red blood cells especially the pyruvate kinase (PKLR) are the key driver for liver colonization. There were evidences suggesting the fact that PKLR promotes the survival of cells in the tumor during the high cell density and deprivation of oxygen.
From sections 2 to 6 it was identified that metastasis of tumour cells is a process comprising of several sequential steps. Metastasis is also driven by genetic alterations and signalling pathways. In reagrds to colon cancer, it can be said that both genetic and epigenetic alterations are common (Campbell, 2004). Genetics play an important role in metastasis as the hereditary non-polyposis colorectal cancer often termed, as HNPCC is responsible for colon cancer prevailing in a family (Tanaz, et al, 2012).
8. Conclusion From the above analysis it may be inferred that metastasis cascade of tumour cells is a complex process. Tumour cells have to progress through several stages before metastasis can be formed in distant organs. Mutations of genes and signalling between genetic pathways promote metastasis. It was also noted that the immune system has a significant impact on almost every step of the metastatic cascade. This means that one of the ways through which metastatic cancer can be treated is through the targeting of immune pathways.
Agarwal E, Brattain MG, Chowdhury S. (2013) Cell survival and metastasis regulation by AKT signaling in colorectal cancer. Cell Signal, Vol.25, pp.1711–1719.
Bayon, L.G., Izquierdo, M.A., Sirovich, I. (1996) Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology, Vol.23, pp.1224-1231.
Balkwill FR, Capasso M, Hagemann T, (2012) The tumor microenvironment at a glance. J Cell Sci, Vol.125, pp.5591–5596.
Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. (2014) Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol, Vol.20, pp. 5679–5684.
Bos JL. (1989) Ras oncogenes in human cancer: a review. Cancer Res, Vol.49, pp.4682–4689.
Braun S, Vogl FD, Naume B. (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med, Vol.353, pp. 793–802.
Braet F, Nagatsuma K, Saito M, Soon L, Wisse E, Matsuura T. (2007) The hepatic sinusoidal endothelial lining and colorectal liver metastases. World J Gastroenterol, Vol.13, pp. 821-825.
Campbell PM, Der CJ. (2004) Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol, Vol.14, pp. 105–114.
Cancer Research UK (2017) Cancer, the blood and circulation. Available at
Chin AR, Wang SE (2016) Cancer tills the premetastatic field: mechanistic basis and clinical implications. Clin Cancer Res, Vol. 22, pp.3725–3733.
Chiang SP, Cabrera RM, Segall JE (2016) Tumor cell intravasation. Am J Physiol Cell Physiol, Vol.311, pp.C1–C14.
Decker, T., Lohmann-Matthes, M.L., Karck, U. (1989) Comparative study of cytotoxicity, tumor necrosis factor and prostaglandin release after stimulation of rat Kupffer cells. Journal of Leukoc Biology, Vol.45, pp.139-146.
Droujinine IA, Eckert MA, Zhao W. (2013) To grab the stroma by the horns: from biology to cancer therapy with mesenchymal stem cells. Oncotarget, Vol.4, pp.651-664.
Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. (2004) Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res, Vol. 64, pp.8492-8495.
Fidler I, J. (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer, Vol.3, pp.453-458.
Fidler IJ (1970) Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 i-5-iodo-2’-deoxyuridine. J Natl Cancer Inst 1970, Vol.45, pp. 773–782.
Guan, X. (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B. Vol. 5, pp. 402–418.
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell, Vol. 144, pp. 646–674.
Haier J, Nicolson GL. (2001) The role of tumor cell adhesion as an important factor in formation of distant colorectal metastasis. Dis Colon Rectum, Vol.44, pp.876-888.
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer; Vol.9, pp.239–252.
Karkkainen MJ, Mäkinen T, Alitalo K. (2002) Lymphatic endothelium: a new frontier of metastasis research. Nat Cell Biol, Vol. 4, pp. E2-E5.
Konstantinos A Paschos, Ali W Majeed, Nigel C.(2014) Natural history of hepatic metastases from colorectal cancer - pathobiological pathways with clinical significance. World J Gastroenterol, Vol. 20(14), pp.3719-3737.
Kaplan RN, Riba RD, Zacharoulis S. (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, Vol.438, pp.820–827.
Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell, Vol.84, pp.359–369.
Lukanidin E, Sleeman JP (2012) Building the niche: the role of the S100 proteins in metastatic growth. Semin Cancer Biol, Vol.22, pp. 216–225.
MSKCC (2016) Treatment for Metastatic Colon Cancer. Available at
Massaguel J, Obenauf A.C. (2016) Metastatic colonization by circulating tumour cells. Journal of Nature, Vol. 529, p. 1.
Mehlen P, Puisieux A. (2006) Metastasis: a question of life or death. Nat Rev Cancer, Vol.6, pp. 449-458.
Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massague J. (2016) Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell, Vol.165, pp.45–60.
Nathan, C.F. (1987) Secretory products of macrophages. Journal of Clinical Investigations, Vol.79, pp.319-326.
Novellasdemunt L, Antas P, Vivian S. W. Li (2015) Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. American Journal of Physiology - Cell Physiology. Vol. 309 no. 8, C511-C521
Nguyen A, Loo JM, Mital R, Weinberg EM, Man FY, Zeng Z, Paty PB, Saltz L, Janjigian YY, de Stanchina E, Tavazoie SF. (2016) PKLR promotes colorectal cancer liver colonization through induction of glutathione synthesis. J Clin Invest. 126(2):681-94.
Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. (1995) Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res, Vol.55, pp.2752-2755.
Pachmayr E, Treese C, Ulrike S. (2017) Underlying mechanisms for distant metastasis – molecular biology. Visceral Medicine, Vol.33, pp.11-20.
Pantel K, Speicher MR (2016) The biology of circulating tumor cells. Oncogene; Vol.35, pp. 1216–1224.
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer Vol. 9, pp. 285–293.
Polivka J Jr, Janku F (2014) Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther, Vol.142, pp.164–175.
Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. (2013) Chemokines in tumor progression and metastasis. Oncotarget, Vol. 4, pp.2171–2185.
Shin MK, Kim SK, Jung H. (2011) Integration of intra- and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab Chip, Vol.11, pp.3880-3887.
Samarasinghe, B. (2013) Hallmarks of Cancer Tissue Invasion and Metastasis. Available at
Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. (2007) High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci USA. Vol. 104, pp.17406-17411.
Shimizu S, Yamada N, Sawada T, Ikeda K, Nakatani K, Seki S, Kaneda K, Hirakawa K. (2000) Ultrastructure of early phase hepatic metastasis of human colon carcinoma cells with special reference to desmosomal junctions with hepatocytes.
Pathol Int, Vol.50, pp.953-959.
Tanaz, A, Wilson, J, Chu Q, Mills, G (2012) Genetic Alterations in Colorectal Cancer. Gastrointestinal Cancer Resv.5 (1)- 19-27.
Thiery JP, Acloque H, Huang RY, Nieto MA. (2009) Epithelial-mesenchymal transitions in development and disease. Cell, Vol.139, pp.871–890.
Tremblay P, Auger F, and Huot J (2006) Regulation of trans-endothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene. 25(50):6563-73. Epub
Tsuji, T., Ibaragi, S., Fu Hu, G. (2009) Epithelial Mesenchymal Transition and Cell Co-operativity in Mestastais. Journal of Cancer Research, Vol.69(18), pp.7135-7139.
Vekemans K, Braet, F. (2005) Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World Journal of Gastroenterology, Vol. 11(33), pp.5095-5102.
Valastyan S, Weinberg R.A. (2011) Tumour metastasis: molecular insights and evolving paradigms. Cell, Vol. 147, pp. 275-292.
Van Zijl, F., Krupitza, G., Mikulits, W. (2011) Initial steps of metastasis : Cell Invasion & Endothelial Transmigration. Journal of Mutation Research, Vol.728, pp.23-34.
Xu, Y, and Pasche B (2007) TGF-β signaling alterations and susceptibility to colorectal cancer. Human Molecular Genetics, Volume 16
Yamauchi K, Yang M, Jiang P, Yamamoto N, Xu M. (2005) Real-time in vivo dual-color imaging of intracapillary cancer cell and nucleus deformation and migration. Cancer Res, Vol. 65, pp. 4246-4252.
Yan M, Jurasz P. (2016) the role of platelets in the tumor microenvironment: from solid tumors to leukemia. Biochim Biophys Acta, Vol.1863, pp.392–400.
Yoo J, Perez CE, Nie W, Sinnett-Smith J, Rozengurt E. (2013) TNFalpha and LPA promote synergistic expression of COX-2 in human colonic myofibroblasts: role of LPA-mediated transactivation of upregulated EGFR. BMC Gastroenterol, Vol.13, p.90.
Zingg U, Montani M, Frey DM, Dirnhofer S, Esterman AJ, Went P, Oertli D. (2010) Tumour-infiltrating lymphocytes and survival in patients with adenocarcinoma of the oesophagus. Eur J Surg Oncol, Vol.36, pp.670–677.
Uniresearchers is a leading team of researchers in the field of academic writing. With the track record of delivering 500+ high quality dissertations and 2500+ essays, assignments and coursework’s Uniresearchers has always tried to keep up with the expectations of our clients.
Uniresearchers is a leading team of researchers in the field of academic writing. With the track record of delivering 500+ high quality dissertations and 2500+ essays, assignments and coursework’s Uniresearchers has always tried to keep up with the expectations of our clients.